









Improve trial performance by up to 30% with monitoring that reduces complexity and keeps your study on track.
Visualize trial data trends, uncover hidden signals, and focus on what matters most with intelligent filtering and automation.
Gain full visibility with dynamic data structures, and prove compliance through a clear, auditable evidence trail.
Automate monitoring tasks, streamline review cycles, and free resources for high-value work.
With OPRA, achieve smarter oversight that saves time, reduces risk, and keeps your trials on track.
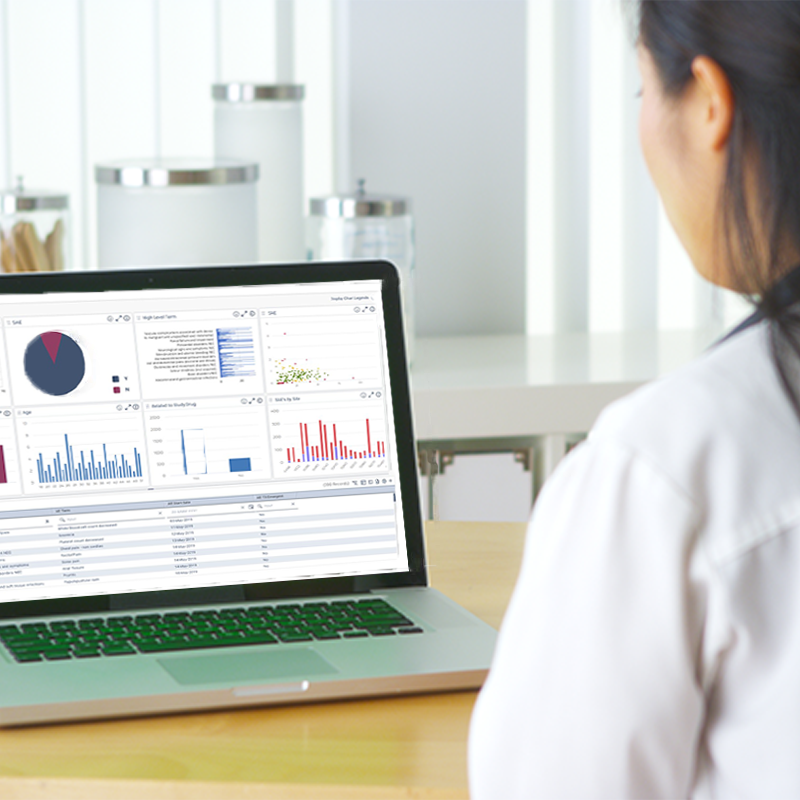

A single command centre for Key Risk Indicators and Quality Tolerance Limits. Track trends, surface insights, and maintain oversight with ease.
Automatically generate and update monitoring observations based on your workflows to ensure consistency across teams.
Analyse data by study, country, site, subject, or endpoint. Spot patterns, investigate anomalies, and act with precision.
Plan, review, and track monitoring activities in one integrated workspace. Maintain alignment across stakeholders and ensure nothing is missed.
Availability of up-to-date risk scores
View evolution of risks over time
Centralized portal to collaborate with stakeholders
Customizable alerts
Customizable data visualizations
Eradicates duplication
Assign actions to stakeholders
Full discoverability of mitigations after closeout
Dependable software. Genuine trust. TRI delivers, on time, at scale, with clarity.
In a rigid industry, we’re the human touch: pragmatic, responsive, and known by name.
We lead, not follow. TRI is fast-moving and data-informed, staying ahead of Sponsor and CRO needs.



