
Mapping the Oncology Research Landscape
Oncology R&D is evolving through precision, disciplined portfolios and pragmatic AI integration.









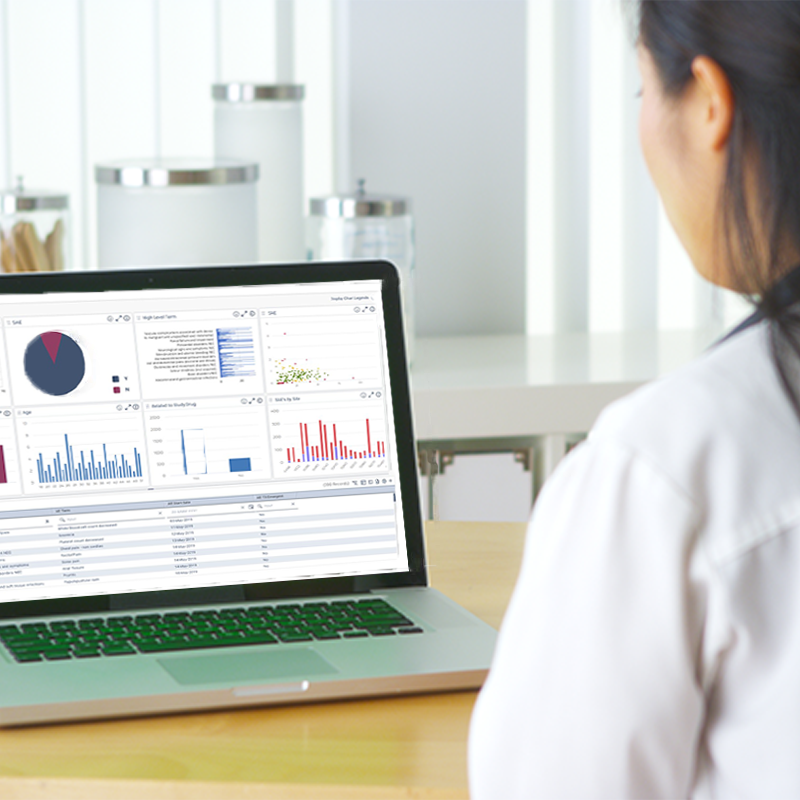
At TRI, we make Risk-Based Quality Management simple and effective, helping sponsors and CROs cut down on manual work, and achieve better clinical outcomes with confidence.
Gain deeper insights and make faster, smarter decisions, that let you analyze from every angle.
Confidently shift to data-driven decisions with adaptive insights that evolve as your trial progresses.
Reduce monitoring costs by up to 30%, allowing your team to focus on what matters most, patient safety.
See how our expertise in risk-based strategies empowers your team to cut costs, stay compliant, and deliver better clinical outcomes.

Learn the principles of Good Clinical Practice, key responsibilities for IRBs and Sponsors, and how RBQM strengthens compliance.
Master the fundamentals of clinical trial design, conduct, and reporting with our comprehensive GCP e-learning course.
Explore how RBQM strengthens clinical trials by identifying critical data, evaluating risks, and improving decision-making.
Turn complex trial metrics into intuitive, actionable visuals.
Monitor, assess, and mitigate risks from one unified platform.
Let OPRA handle the manual tasks, so your team can focus on strategy.
Streamline operations and reclaim time with intelligent trial oversight.
Proven performance across hundreds of global studies.
OPRA fits effortlessly into your tech stack without disruption.
Built to meet the highest standards in clinical data integrity and security.
Flexible, scalable, and ready to support your study from start to finish.
Dependable software. Genuine trust. TRI delivers, on time, at scale, with clarity.
In a rigid industry, we’re the human touch: pragmatic, responsive, and known by name.
We lead, not follow. TRI is fast-moving and data-informed, staying ahead of Sponsor and CRO needs.
As clinical trials evolve, quality and risk‑management approaches must keep pace. SCOPE’s Quality & Monitoring stream brings together clinical operations and RBQM experts to share proven best practices, real‑world case studies, and actionable strategies for embedding a risk‑based approach across the clinical research lifecycle.
You’ll find the TRI team at Booth #1114





Oncology R&D is evolving through precision, disciplined portfolios and pragmatic AI integration.

A deeper look at the recurring FDA warning letter findings that reveal whether your computer system validation is truly under control.

Clinical trials may look stable on paper but rising rare disease, CNS and cardiovascular complexity is exposing old operating models.